After five decades of campaigning, people who were allegedly damaged by a pregnancy drug called Primodos had looked like they were about to get recognition of their condition.
Known as “the forgotten thalidomide”, many mothers who used hormone pregnancy tests had children born with a range of conditions including shortened limbs, spinal problems and heart defects.
Earlier this month, the Department of Work and Pensions signed off a document which was intended to be used by assessors for disability support – Personal Independence Payment (PIP) – explaining that damage from hormone pregnancy tests (HPT) is a “recognised condition”.
The DWP’s Condition Insight Report, similar to one produced for thalidomide victims, was originally due to be published, for internal use by assessors, on 3 June and stated: “HPT (hormone pregnancy test) associated damage is seen as a recognised condition.”
It added: “Evidence has since been found to link the use of the drug to congenital malformations.
“As the scientific context was disputed for many years, many survivors, their careers and families felt they have been severely failed by state agencies in recognising their condition.”
It seems, however, that the group is facing more disappointment.
When Sky News called the DWP for a comment on 1 June, two days before the release date for the document, it was suddenly put back a week. A week later it was pushed back again to an unknown date.
A cross-party group of MPs held an emergency meeting and has now written to the Work and Pensions Secretary Therese Coffey demanding to know what is going on.
Yasmin Qureshi MP, who represents the group, wrote: “To withdraw or change its content would add insult to injury to the hundreds of families who have been affected by hormone pregnancy tests.”
But it seems that is what is happening, as the department is now making reference to a review which contested the claim that the drug was damaging.
A DWP spokesperson told Sky News: “The government respects the findings of the 2017 review by the Independent Expert Working Group (EWG) of the Commission on Human Medicines, which concluded the available scientific evidence does not support a causal association between hormone pregnancy tests and adverse outcomes of pregnancy.
“This does not prevent people from applying for health and disability benefits and we are absolutely committed to helping people get the support they are entitled to.”
It raises questions as to why the DWP produced a condition report into a condition it now says it doesn’t recognise.
Marie Lyon, chair of the Association for Children Damaged By Hormone Pregnancy Tests, told Sky News: “The whole thing has been an absolute disaster. When the DWP consider that they are talking about people with life-changing difficulties, it’s so distressing they have backtracked.
“And it seems to me that other people outside of the department have had an influence in this decision and I want to know who they are.”
Last year, the alleged victims began the process of legal action against the government and the manufacturer over the alleged failure to remove the product from the market after concerns had been raised as early as 1967.
In 2017, a commissioned government review did indeed say there was not enough evidence to prove a causal association between the drug and malformations in the womb.
But the findings of this review have since been contested by a group of epidemiologists at Oxford University.
A Sky News documentary released in 2017 produced new evidence of a potential link and also uncovered documents suggesting some key findings had been covered up by a UK regulator back in the 1970s.
From 1958 to 1978, over a million women in the UK used hormone pregnancy tests. Some studies suggest the high proportion of women who used these drugs went on to have malformations or even miscarriages, but the science is contested.
The most commonly used in the UK, manufactured by Schering Chemicals, was called Primodos. It was prescribed by GPs or given as free samples by the drug company.
Primodos was marketed as more convenient than other available pregnancy tests and contained hormones including high doses of oestrogen ethinylestradiol and the progestogen norethisterone.
Experts say the quantities amounted to 40 times the levels found in today’s oral contraceptives.
The manufacturer, which was taken over by Bayer AG in 2016, has always denied an association between the drug and malformations.
An independent review, led by Baroness Cumberlege, is due to report on the subject next month.
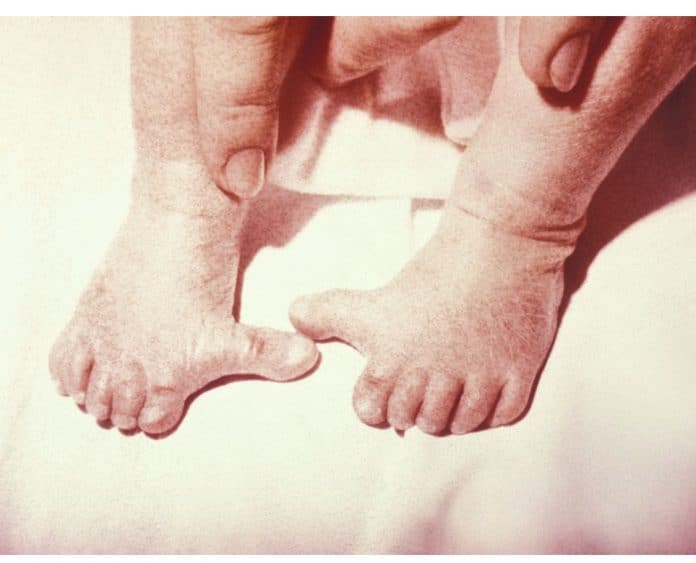






I was prescribed Primodos in September 1974. A son born with mutilated abnormalities in May 1975. In 1976 again prescribed Primodos to ammenhoria to regulate mo they periods. This time a warning on the package stated, ‘Do not take if Pregnant due to abnormalities to the unborn child, miscarriages and deaths to many thousands of unborn children. How can the government and Drug Company use the same drug for two different applications. To diagnose a pregnancy and to abort a foetuses in my case in 1976 to bring on a monthly period ie a abortion if pregnant. I filled Schering Chemicals for damages to my son in 1977. They offered an award in January of 1978. This was because one month before the Association for Children Damaged by Hormone Pregnancy Test I was in the process of organising in February 1978. I refused their offer on moral grounds as I would not be in the position to share the news of this offer to other victims and families. The offer was not in writing but conveyed on the phone from Schering’s to my Solicitor at the time.